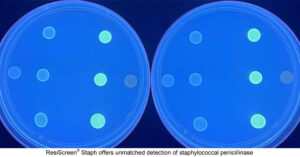
 Lyon, France — Octobre 21, 2025 — Molsid today announced the successful completion of the clinical performance study for its first diagnostic kit, ResiScreen® Staph. This milestone marks a major step toward the commercial launch of the company’s innovative solution for the reliable detection of β-lactam resistance in Staphylococcus strains.
Lyon, France — Octobre 21, 2025 — Molsid today announced the successful completion of the clinical performance study for its first diagnostic kit, ResiScreen® Staph. This milestone marks a major step toward the commercial launch of the company’s innovative solution for the reliable detection of β-lactam resistance in Staphylococcus strains.
The study was conducted in collaboration with the French National Reference Centre for Staphylococci (Hospices Civils de Lyon, France) under the supervision of Prof. Frédéric Laurent and Dr. Céline Dupieux-Chabert. It evaluated the performance of ResiScreen® Staph on 300 clinical isolates of Staphylococcus aureus and Staphylococcus non-aureus, confirming the kit’s robustness, reliability, and clinical relevance.